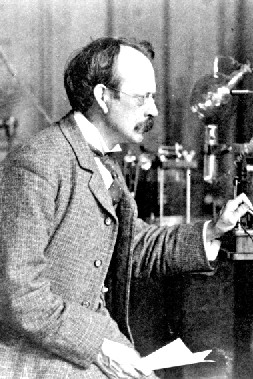-
(b.) -1856 December 18(d.)1940 August 30
Bio/Description
A British physicist and Nobel laureate, he was born in Cheetham Hill, Manchester, England. His early education took place in small private schools where he demonstrated great talent and interest in science. In 1870 he was admitted to Owens College at the unusually young age of 14. His parents planned to enroll him as an apprentice engineer to Sharp-Stewart & Co., a locomotive manufacturer, but these plans were cut short when his father died in 1873. He moved on to Trinity College, Cambridge in 1876. In 1880, he obtained his BA in mathematics (Second Wrangler and 2nd Smith's prize) and MA (with Adams Prize) in 1883. In 1884 he became Cavendish Professor of Physics. One of his greatest contributions to modern science was in his role as a highly gifted teacher, as seven of his research assistants and his aforementioned son won Nobel Prizes in physics. His son won the Nobel Prize in 1937 for proving the wavelike properties of electrons. He was awarded a Nobel Prize in 1906, "in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases." He was knighted in 1908 and appointed to the Order of Merit in 1912. In 1914 he gave the Romanes Lecture in Oxford on "The atomic theory". In 1918 he became Master of Trinity College, Cambridge, where he remained until his death in 1940, and was buried in Westminster Abbey, close to Sir Isaac Newton. He was elected a Fellow of the Royal Society on June 12, 1884 and was subsequently President of the Royal Society from 1915 to 1920. Through his explorations on the properties of cathode rays, he was the first to suggest that the fundamental unit was over 1000 times smaller than an atom, suggesting the sub-atomic particles now known as electrons. He made his suggestion on April 30, 1897 following his discovery that Lenard rays could travel much further through air than expected for an atomic-sized particle. He concluded that the rays were composed of very light, negatively charged particles which were a universal building block of atoms. He called the particles "corpuscles", but later scientists preferred the name electron which had been suggested by George Johnstone Stoney earlier in 1894. He believed that the corpuscles emerged from the atoms of the trace gas inside his cathode ray tubes (now used in televisions, computer monitors and electron microscopes). He thus concluded that atoms were divisible, and that the corpuscles were their building blocks. To explain the overall neutral charge of the atom, he proposed that the corpuscles were distributed in a uniform sea of positive charge; this was the "plum pudding" model?the electrons were embedded in the positive charge like plums in a plum pudding.
-
Date of Birth:
1856 December 18 -
Date of Death:
1940 August 30 -
Gender:
Male -
Noted For:
Discovered the electron and isotopes, inventor of the mass spectrometer and his experimentation of cathode rays ultimately used in computer monitors -
Category of Achievement:
-
More Info: